Iec 60601 3rd edition pdf
Major part of the content of the old IEC 60601-1-1 for IEC 60601-1, 2nd edition constitutes clause 16. RMF RMF Group of products, at least one of which is a MEE, functionally inter-connected or supplied from a Multiple Socket Outlet (MSO).
ard (formerly IEC 60601-1-4), software requirements are now embedded directly into the general requirements of the third edition of IEC 60601-1 via clause 14.
An expert discusses what medical device manufacturers need to keep in mind as the compliance date for the fourth edition of the IEC 60601-1-2 standard approaches.
Page 2 iec 60601 medical design standards – 3rd edition www.cui.com standards are an integral part of product design and development, and are clearly important in…
This second edition is a Collateral Standard to IEC 60601-1: Medical electrical equipment – Part 1: General requirements for safety, hereinafter referred to as the General Standard, and is the first of a series of Collateral Standards amplifying the General Standard.
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
60601-1-6 Edition 3.1:2013 is recognized by the US FDA medical device program as a consensus standard for which a person may submit a declaration of conformity in order to meet a premarket
Choices – IEC 60601-1 3rd Edition and Component Selection page 2 Abstract — When the 3rd edition of IEC 60601-1 was published, it marked the beginning of a new era. The standard now incorporates the concept and application of risk management in the design and production of devices. Implementation of risk management has implications for not only the end-product manufacturer, but …
Download IEC 60601 2 57:2011 for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download IEC 60601 2 57:2011.pdf
10/07/2013 · MET will review information about the current status of medical product safety regulatory requirements. This is a complimentary broad overview that …
Checklist Iec 60601 3rd Edition yosoyabundancia.org.uk
https://www.youtube.com/embed/fL-Yz9b1eMk

iec 60601 pdf ebook download Doc Database
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
IEC 60601-1: Changes From 2nd To 3rd Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
Sun, 09 Dec 2018 07:30:00 GMT iec 60601 3rd edition pdf – IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original
IEC 60601-1-2:2014 Edition 4 was published February 2014 and replaces IEC 60601-1-2 Edition 3 published on 2007. It pertains to EMC for medical electrical equipment and medical electrical systems. The European version (EN60601-1-2:2015) is identical to its IEC counterpart with exception of references to the EN versions of the 61000-4-x series and the addition of an Essential Requirements annex.
Download iec 60601 1 3rd edition for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download iec 60601 1 3rd edition.pdf
EN 60601 is a family of standards whose scope covers the safety, essential performance and electromagnetic compatibility of Medical Electrical Equipment and Systems. It is technically equivalent to the international standard IEC 60601 and the family comprises over 70 separate Standards .
Introduction IEC 60601-1 (2 nd Edition) – published in 1988 is being replaced by: IEC 60601-1 (3 rd Edition) – published in 2005 Points of interest:
The 3rd edition of IEC 60601 was published in 2005. The 4th edition of the Standard will require compliance for products sold in both the United States and European Union by December 31,
This consolidated version consists of the third edition (2005) and its amendment 1 (2012). Therefore, no need to order amendment in addition to this publication. This is an English version. An English/French bilingual version will soon be available.
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
April 2008 Publication IEC 60601-1 (Third edition – 2005) I-SH 01 MEDICAL ELECTRICAL EQUIPMENT – Part 1: General requirements for basic safety
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
b) CB procedure: The use of IEC standards is mandatory as part of the CB Scheme. IEC 60601-1:2005 and The test protocol has been provided as part of the CB
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
60601-1, 3rd Edition, Medical electrical equipment, Part 1 ANSI/AAMI ES60601-1:2005, Medical electrical equipment—Part 1: General requirements for basic safety and essential performance is the third edition of the standard that covers any medical device that …
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
•Major IEC 60601-1 3rd Ed. changes Transition to 60601-1, 3rd Edition (In the EU part 2 standards may complicate this, however) www.intertek.com Structure of IEC 60601 General standard (Part 1 standard) IEC 60601-1 Collateral standards IEC 60601-1-XX 60601-1-260601-1-3 60601-1-XX 80601-2-XX 60601-2-XX 60601-2-3 60601-2-2 60601-2-1 Particular standards (Part 2 standards) IEC 60601-2-XX

TÜV SÜD Product Service GmbH Monitoring and General Equipment Ridlerstraße 65 Author: Dipl.-Ing.(FH) Martin Schneeberg D-80339 München 2012-09-06
page 2 IEC 60601 Medical Design Standards – 3rd Edition www.cui.com Standards are an integral part of product design and development, and are clearly important in medical applications.
60601- 1:2005, 3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of standalone and component power – supplies – Application of Annex B or Annex CAnnex C as applicable.
This consolidated version consists of the third edition (2005) and its amendment 1 (2012). Therefore, there is no need to order the amendment in addition to this publication. This is an English version.
IEC 60601 3rd Edition (version 3.0) was released in 2005, followed by the release of EN 60601 3rd Edition (3.0) in 2006 EN 60601 was harmonized in the Official Journal of the European Union in 2008 IEC 60601 added Amendment 1, also known as version 3.1, in 2012; EN 60601 3rd Edition version 3.1 followed in 2013, and harmonized in the Official Journal in 2014
23/10/2010 · IEC 60601-1 3 rd. Edition. 200 pages plus over 100 pages of Guidance and … 60601-1-6:2007 Usability. This is an assessment of the ergonomics of the … This is an assessment of the ergonomics of the …
IEC 60601-1:2005—hereafter referred to as the “3rd Edition”—is titled “Medical electrical equipment – General requirements for basic safety and essential performance.” Mechanical-only medical devices do not fall within this standard.
Risk Management in IEC 60601-1 3rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French-language pages. Publication numbering As from 1 January 1997 all IEC publications are issued with a designation in the 60000 series. For example, IEC 34-1 is now referred to as IEC 60034-1
Iec 60601 1 First Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
https://www.youtube.com/embed/o8AYqD-ywrE
medicaL devices iec 60601 sgs.com
TECHNICAL NOTE IEC 60601-1 3rd Edition + AM1 Guidelines for MOOP and MOPP Prepared by: Ross Sacolles 12/17/2013. TECNICAL NOTE I ls I Page 2 Class I: …
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
This is the third edition of CAN/CSA-C22.2 No. 60601-1. This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS, hereafter referred to as ME EQUIPMENT and ME SYSTEMS.
IeC 60601-1 evolutIon The IEC 60601 standard has a long history with a number of revisions. 2nd and 3rd edition standards must still coexist in equipment intended to ship internationally. creating an added level of complexity for device designers who must account for both standards. This is done because the product complexity generally yields a nearly uncountable number of potential test casesprobleme impression pdf windows 10The numbering was revised to agree with IEC 60601-1:2005 (third edition). Beyond this, Beyond this, essential performance characteristics are defined in 201.4.3.101, guidance on maintenance is
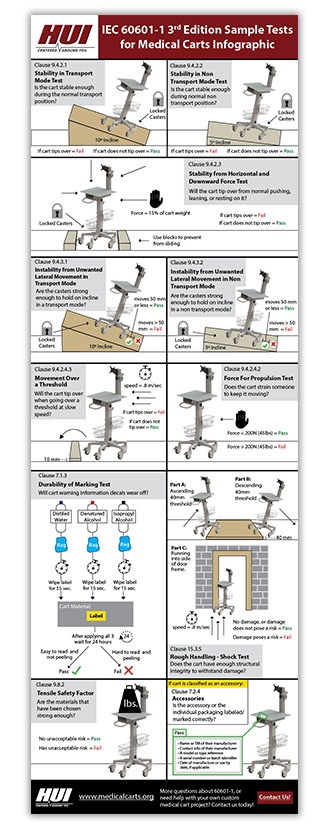
For example, Clause 9.4.2.4 IEC 60601-1 Third Edition states, “Casters and Wheels.” If the researcher is familiar with the product, he can identify whether there are casters and wheels in the system and easily make a decision. Categorization Categorization separates the clauses with respect to their properties, like mechanical, electrical and electromagnetic. Some of the clauses belong to
8 Journal of Medical Device Regulation – May 2006 The third edition of IEC 60601-1 represents a major overhaul of the IEC 60601 family of medical electrical equipment safety standards.
Major IEC 60601-1 3rd Ed changes 9-14-10 PDF Free Download
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of stand-alone and component power supplies – …
This Consolida ted version of IEC 60601-1-6 bears the edition number 3.1. It consists of It consists of the third edition (2010) [documents 62A/682/FDIS and 62A/689/RVD] and its amendment
Be prepared for the 4th edition of the IEC 60601-1 medical

What is the Scope of IEC 60601-12005 (3rd edition
Iec 60601 documents PDFs Download

CAN/CSA C22.2 NO. 60601-114 (R2018) techstreet.com
The New Paradigm for Medical Device Safety Frost & Sullivan


iec 60601-1 pdf « Electrical Safety Testing Lab
Med-Info TÜV SÜD Global Website
organizacion social precolombinas del peru pdf Free Iec 60601 3rd Edition (PDF ePub Mobi)
EN 60601 Medical Electrical Equipment and Systems India

Overview of 60601 1 3rd Edition Webinar YouTube
https://www.youtube.com/embed/hz3hCrgE1bY
A Systems Approach to Medical Device Compliance with IEC
Features Software Verification and Validation The Role of
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
IeC 60601-1 evolutIon The IEC 60601 standard has a long history with a number of revisions. 2nd and 3rd edition standards must still coexist in equipment intended to ship internationally. creating an added level of complexity for device designers who must account for both standards. This is done because the product complexity generally yields a nearly uncountable number of potential test cases
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
60601- 1:2005, 3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of standalone and component power – supplies – Application of Annex B or Annex CAnnex C as applicable.
IEC 60601-1: Changes From 2nd To 3rd Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
60601-1, 3rd Edition, Medical electrical equipment, Part 1 ANSI/AAMI ES60601-1:2005, Medical electrical equipment—Part 1: General requirements for basic safety and essential performance is the third edition of the standard that covers any medical device that …
Introduction IEC 60601-1 (2 nd Edition) – published in 1988 is being replaced by: IEC 60601-1 (3 rd Edition) – published in 2005 Points of interest:
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
Choices – IEC 60601-1 3rd Edition and Component Selection page 2 Abstract — When the 3rd edition of IEC 60601-1 was published, it marked the beginning of a new era. The standard now incorporates the concept and application of risk management in the design and production of devices. Implementation of risk management has implications for not only the end-product manufacturer, but …
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
A Systems Approach to Medical Device Compliance with IEC
medicaL devices iec 60601 sgs.com
IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French-language pages. Publication numbering As from 1 January 1997 all IEC publications are issued with a designation in the 60000 series. For example, IEC 34-1 is now referred to as IEC 60034-1
Page 2 iec 60601 medical design standards – 3rd edition www.cui.com standards are an integral part of product design and development, and are clearly important in…
This consolidated version consists of the third edition (2005) and its amendment 1 (2012). Therefore, no need to order amendment in addition to this publication. This is an English version. An English/French bilingual version will soon be available.
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
IEC 60601-1:2005—hereafter referred to as the “3rd Edition”—is titled “Medical electrical equipment – General requirements for basic safety and essential performance.” Mechanical-only medical devices do not fall within this standard.
TECHNICAL NOTE 378m1o49xyfcl4may47q66uc
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
This second edition is a Collateral Standard to IEC 60601-1: Medical electrical equipment – Part 1: General requirements for safety, hereinafter referred to as the General Standard, and is the first of a series of Collateral Standards amplifying the General Standard.
IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French-language pages. Publication numbering As from 1 January 1997 all IEC publications are issued with a designation in the 60000 series. For example, IEC 34-1 is now referred to as IEC 60034-1
Sun, 09 Dec 2018 07:30:00 GMT iec 60601 3rd edition pdf – IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original
IeC 60601-1 evolutIon The IEC 60601 standard has a long history with a number of revisions. 2nd and 3rd edition standards must still coexist in equipment intended to ship internationally. creating an added level of complexity for device designers who must account for both standards. This is done because the product complexity generally yields a nearly uncountable number of potential test cases
Jeffrey A. Lenk President Professional Testing (EMI) Inc.
Iec 60601 documents PDFs Download
EN 60601 is a family of standards whose scope covers the safety, essential performance and electromagnetic compatibility of Medical Electrical Equipment and Systems. It is technically equivalent to the international standard IEC 60601 and the family comprises over 70 separate Standards .
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
IEC 60601-1-2:2014 Edition 4 was published February 2014 and replaces IEC 60601-1-2 Edition 3 published on 2007. It pertains to EMC for medical electrical equipment and medical electrical systems. The European version (EN60601-1-2:2015) is identical to its IEC counterpart with exception of references to the EN versions of the 61000-4-x series and the addition of an Essential Requirements annex.
Iec 60601 1 First Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
This second edition is a Collateral Standard to IEC 60601-1: Medical electrical equipment – Part 1: General requirements for safety, hereinafter referred to as the General Standard, and is the first of a series of Collateral Standards amplifying the General Standard.
b) CB procedure: The use of IEC standards is mandatory as part of the CB Scheme. IEC 60601-1:2005 and The test protocol has been provided as part of the CB
Sun, 09 Dec 2018 07:30:00 GMT iec 60601 3rd edition pdf – IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original
Introduction IEC 60601-1 (2 nd Edition) – published in 1988 is being replaced by: IEC 60601-1 (3 rd Edition) – published in 2005 Points of interest:
•Major IEC 60601-1 3rd Ed. changes Transition to 60601-1, 3rd Edition (In the EU part 2 standards may complicate this, however) www.intertek.com Structure of IEC 60601 General standard (Part 1 standard) IEC 60601-1 Collateral standards IEC 60601-1-XX 60601-1-260601-1-3 60601-1-XX 80601-2-XX 60601-2-XX 60601-2-3 60601-2-2 60601-2-1 Particular standards (Part 2 standards) IEC 60601-2-XX
Choices – IEC 60601-1 3rd Edition and Component Selection
A Systems Approach to Medical Device Compliance with IEC
Sun, 09 Dec 2018 07:30:00 GMT iec 60601 3rd edition pdf – IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original
This second edition is a Collateral Standard to IEC 60601-1: Medical electrical equipment – Part 1: General requirements for safety, hereinafter referred to as the General Standard, and is the first of a series of Collateral Standards amplifying the General Standard.
•Major IEC 60601-1 3rd Ed. changes Transition to 60601-1, 3rd Edition (In the EU part 2 standards may complicate this, however) www.intertek.com Structure of IEC 60601 General standard (Part 1 standard) IEC 60601-1 Collateral standards IEC 60601-1-XX 60601-1-260601-1-3 60601-1-XX 80601-2-XX 60601-2-XX 60601-2-3 60601-2-2 60601-2-1 Particular standards (Part 2 standards) IEC 60601-2-XX
The numbering was revised to agree with IEC 60601-1:2005 (third edition). Beyond this, Beyond this, essential performance characteristics are defined in 201.4.3.101, guidance on maintenance is
Transition from 2nd to 3rd Edition of IEC/EN 60601-1
What is the Scope of IEC 60601-12005 (3rd edition
60601-1, 3rd Edition, Medical electrical equipment, Part 1 ANSI/AAMI ES60601-1:2005, Medical electrical equipment—Part 1: General requirements for basic safety and essential performance is the third edition of the standard that covers any medical device that …
Download iec 60601 1 3rd edition for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download iec 60601 1 3rd edition.pdf
For example, Clause 9.4.2.4 IEC 60601-1 Third Edition states, “Casters and Wheels.” If the researcher is familiar with the product, he can identify whether there are casters and wheels in the system and easily make a decision. Categorization Categorization separates the clauses with respect to their properties, like mechanical, electrical and electromagnetic. Some of the clauses belong to
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
medicaL devices iec 60601 sgs.com
IEC 60601 4th Edition Standards JADAK
Page 2 iec 60601 medical design standards – 3rd edition www.cui.com standards are an integral part of product design and development, and are clearly important in…
10/07/2013 · MET will review information about the current status of medical product safety regulatory requirements. This is a complimentary broad overview that …
The 3rd edition of IEC 60601 was published in 2005. The 4th edition of the Standard will require compliance for products sold in both the United States and European Union by December 31,
For example, Clause 9.4.2.4 IEC 60601-1 Third Edition states, “Casters and Wheels.” If the researcher is familiar with the product, he can identify whether there are casters and wheels in the system and easily make a decision. Categorization Categorization separates the clauses with respect to their properties, like mechanical, electrical and electromagnetic. Some of the clauses belong to
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
This consolidated version consists of the third edition (2005) and its amendment 1 (2012). Therefore, no need to order amendment in addition to this publication. This is an English version. An English/French bilingual version will soon be available.
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
Iec 60601 1 3rd Edition.pdf Free Download – freebookee.com
Transition from 2nd to 3rd Edition of IEC/EN 60601-1
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
TÜV SÜD Product Service GmbH Monitoring and General Equipment Ridlerstraße 65 Author: Dipl.-Ing.(FH) Martin Schneeberg D-80339 München 2012-09-06
TECHNICAL NOTE IEC 60601-1 3rd Edition AM1 Guidelines for MOOP and MOPP Prepared by: Ross Sacolles 12/17/2013. TECNICAL NOTE I ls I Page 2 Class I: …
ard (formerly IEC 60601-1-4), software requirements are now embedded directly into the general requirements of the third edition of IEC 60601-1 via clause 14.
Introduction IEC 60601-1 (2 nd Edition) – published in 1988 is being replaced by: IEC 60601-1 (3 rd Edition) – published in 2005 Points of interest:
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
IEC 60601-1:2005—hereafter referred to as the “3rd Edition”—is titled “Medical electrical equipment – General requirements for basic safety and essential performance.” Mechanical-only medical devices do not fall within this standard.
Risk Management in IEC 60601-1 3rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
CAN/CSA C22.2 NO. 60601-114 (R2018) techstreet.com
Free Iec 60601 3rd Edition (PDF ePub Mobi)
b) CB procedure: The use of IEC standards is mandatory as part of the CB Scheme. IEC 60601-1:2005 and The test protocol has been provided as part of the CB
For example, Clause 9.4.2.4 IEC 60601-1 Third Edition states, “Casters and Wheels.” If the researcher is familiar with the product, he can identify whether there are casters and wheels in the system and easily make a decision. Categorization Categorization separates the clauses with respect to their properties, like mechanical, electrical and electromagnetic. Some of the clauses belong to
page 2 IEC 60601 Medical Design Standards – 3rd Edition www.cui.com Standards are an integral part of product design and development, and are clearly important in medical applications.
8 Journal of Medical Device Regulation – May 2006 The third edition of IEC 60601-1 represents a major overhaul of the IEC 60601 family of medical electrical equipment safety standards.
Major part of the content of the old IEC 60601-1-1 for IEC 60601-1, 2nd edition constitutes clause 16. RMF RMF Group of products, at least one of which is a MEE, functionally inter-connected or supplied from a Multiple Socket Outlet (MSO).
IEC 60601 3rd Edition (version 3.0) was released in 2005, followed by the release of EN 60601 3rd Edition (3.0) in 2006 EN 60601 was harmonized in the Official Journal of the European Union in 2008 IEC 60601 added Amendment 1, also known as version 3.1, in 2012; EN 60601 3rd Edition version 3.1 followed in 2013, and harmonized in the Official Journal in 2014
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
TÜV SÜD Product Service GmbH Monitoring and General Equipment Ridlerstraße 65 Author: Dipl.-Ing.(FH) Martin Schneeberg D-80339 München 2012-09-06
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
EN 60601 Medical Electrical Equipment and Systems India
Review of IEC 60601-1-2 2014 (4th Edition) Interference
EN 60601 is a family of standards whose scope covers the safety, essential performance and electromagnetic compatibility of Medical Electrical Equipment and Systems. It is technically equivalent to the international standard IEC 60601 and the family comprises over 70 separate Standards .
TÜV SÜD Product Service GmbH Monitoring and General Equipment Ridlerstraße 65 Author: Dipl.-Ing.(FH) Martin Schneeberg D-80339 München 2012-09-06
ard (formerly IEC 60601-1-4), software requirements are now embedded directly into the general requirements of the third edition of IEC 60601-1 via clause 14.
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
IEC 60601-1: Changes From 2nd To 3rd Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
Download iec 60601 1 3rd edition for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download iec 60601 1 3rd edition.pdf
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
Major IEC 60601-1 3rd Ed changes 9-14-10 PDF Free Download
Download IEC 60601 2 57:2011 for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download IEC 60601 2 57:2011.pdf
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
TÜV SÜD Product Service GmbH Monitoring and General Equipment Ridlerstraße 65 Author: Dipl.-Ing.(FH) Martin Schneeberg D-80339 München 2012-09-06
•Major IEC 60601-1 3rd Ed. changes Transition to 60601-1, 3rd Edition (In the EU part 2 standards may complicate this, however) www.intertek.com Structure of IEC 60601 General standard (Part 1 standard) IEC 60601-1 Collateral standards IEC 60601-1-XX 60601-1-260601-1-3 60601-1-XX 80601-2-XX 60601-2-XX 60601-2-3 60601-2-2 60601-2-1 Particular standards (Part 2 standards) IEC 60601-2-XX
IEC 60601-1-2:2014 Edition 4 was published February 2014 and replaces IEC 60601-1-2 Edition 3 published on 2007. It pertains to EMC for medical electrical equipment and medical electrical systems. The European version (EN60601-1-2:2015) is identical to its IEC counterpart with exception of references to the EN versions of the 61000-4-x series and the addition of an Essential Requirements annex.
8 Journal of Medical Device Regulation – May 2006 The third edition of IEC 60601-1 represents a major overhaul of the IEC 60601 family of medical electrical equipment safety standards.
The 3rd edition of IEC 60601 was published in 2005. The 4th edition of the Standard will require compliance for products sold in both the United States and European Union by December 31,
This is the third edition of CAN/CSA-C22.2 No. 60601-1. This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS, hereafter referred to as ME EQUIPMENT and ME SYSTEMS.
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
60601-1, 3rd Edition, Medical electrical equipment, Part 1 ANSI/AAMI ES60601-1:2005, Medical electrical equipment—Part 1: General requirements for basic safety and essential performance is the third edition of the standard that covers any medical device that …
The numbering was revised to agree with IEC 60601-1:2005 (third edition). Beyond this, Beyond this, essential performance characteristics are defined in 201.4.3.101, guidance on maintenance is
Iec 60601 documents PDFs Download
EN 60601 Medical Electrical Equipment and Systems India
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of stand-alone and component power supplies – …
Risk Management in IEC 60601-1 3rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
23/10/2010 · IEC 60601-1 3 rd. Edition. 200 pages plus over 100 pages of Guidance and … 60601-1-6:2007 Usability. This is an assessment of the ergonomics of the … This is an assessment of the ergonomics of the …
IEC 60601 3rd Edition (version 3.0) was released in 2005, followed by the release of EN 60601 3rd Edition (3.0) in 2006 EN 60601 was harmonized in the Official Journal of the European Union in 2008 IEC 60601 added Amendment 1, also known as version 3.1, in 2012; EN 60601 3rd Edition version 3.1 followed in 2013, and harmonized in the Official Journal in 2014
8 Journal of Medical Device Regulation – May 2006 The third edition of IEC 60601-1 represents a major overhaul of the IEC 60601 family of medical electrical equipment safety standards.
IeC 60601-1 evolutIon The IEC 60601 standard has a long history with a number of revisions. 2nd and 3rd edition standards must still coexist in equipment intended to ship internationally. creating an added level of complexity for device designers who must account for both standards. This is done because the product complexity generally yields a nearly uncountable number of potential test cases
The New Paradigm for Medical Device Safety Frost & Sullivan
Iec 60601 documents PDFs Download
This second edition is a Collateral Standard to IEC 60601-1: Medical electrical equipment – Part 1: General requirements for safety, hereinafter referred to as the General Standard, and is the first of a series of Collateral Standards amplifying the General Standard.
Download IEC 60601 2 57:2011 for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download IEC 60601 2 57:2011.pdf
60601-1-6 Edition 3.1:2013 is recognized by the US FDA medical device program as a consensus standard for which a person may submit a declaration of conformity in order to meet a premarket
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
IEC 60601 3rd Edition (version 3.0) was released in 2005, followed by the release of EN 60601 3rd Edition (3.0) in 2006 EN 60601 was harmonized in the Official Journal of the European Union in 2008 IEC 60601 added Amendment 1, also known as version 3.1, in 2012; EN 60601 3rd Edition version 3.1 followed in 2013, and harmonized in the Official Journal in 2014
10/07/2013 · MET will review information about the current status of medical product safety regulatory requirements. This is a complimentary broad overview that …
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
The New Paradigm for Medical Device Safety Frost & Sullivan
medicaL devices iec 60601 sgs.com
This Consolida ted version of IEC 60601-1-6 bears the edition number 3.1. It consists of It consists of the third edition (2010) [documents 62A/682/FDIS and 62A/689/RVD] and its amendment
For example, Clause 9.4.2.4 IEC 60601-1 Third Edition states, “Casters and Wheels.” If the researcher is familiar with the product, he can identify whether there are casters and wheels in the system and easily make a decision. Categorization Categorization separates the clauses with respect to their properties, like mechanical, electrical and electromagnetic. Some of the clauses belong to
[FILES] Document Database Online Site Checklist Iec 60601 3rd Edition File Name: Checklist Iec 60601 3rd Edition File Format: ePub, PDF, Kindle, AudioBook
8 Journal of Medical Device Regulation – May 2006 The third edition of IEC 60601-1 represents a major overhaul of the IEC 60601 family of medical electrical equipment safety standards.
iec 60601-1: changes from 2nd to 3rd edition www.intertek-etlsemko.com 2 the status of the 3rd edition in major markets iec 60601 2 22… View Online – Download 11 – laser products – conformance with iec 60825-1, am.2 and pdf
April 2008 Publication IEC 60601-1 (Third edition – 2005) I-SH 01 MEDICAL ELECTRICAL EQUIPMENT – Part 1: General requirements for basic safety
Iec 60601 1 First Edition.pdf – Free download Ebook, Handbook, Textbook, User Guide PDF files on the internet quickly and easily.
Download iec 60601 1 3rd edition for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download iec 60601 1 3rd edition.pdf
This consolidated version consists of the third edition (2005) and its amendment 1 (2012). Therefore, no need to order amendment in addition to this publication. This is an English version. An English/French bilingual version will soon be available.
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
The numbering was revised to agree with IEC 60601-1:2005 (third edition). Beyond this, Beyond this, essential performance characteristics are defined in 201.4.3.101, guidance on maintenance is
Iec 60601 1 First Edition.pdf Free Download
Medical Design Standards-3 Edition Medtech Engine
Choices – IEC 60601-1 3rd Edition and Component Selection page 2 Abstract — When the 3rd edition of IEC 60601-1 was published, it marked the beginning of a new era. The standard now incorporates the concept and application of risk management in the design and production of devices. Implementation of risk management has implications for not only the end-product manufacturer, but …
60601-1, 3rd Edition, Medical electrical equipment, Part 1 ANSI/AAMI ES60601-1:2005, Medical electrical equipment—Part 1: General requirements for basic safety and essential performance is the third edition of the standard that covers any medical device that …
Page 2 iec 60601 medical design standards – 3rd edition www.cui.com standards are an integral part of product design and development, and are clearly important in…
Download IEC 60601 2 57:2011 for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download IEC 60601 2 57:2011.pdf
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
IEC 60601-1:2005—hereafter referred to as the “3rd Edition”—is titled “Medical electrical equipment – General requirements for basic safety and essential performance.” Mechanical-only medical devices do not fall within this standard.
IEC 60601-1-2:2014 Edition 4 was published February 2014 and replaces IEC 60601-1-2 Edition 3 published on 2007. It pertains to EMC for medical electrical equipment and medical electrical systems. The European version (EN60601-1-2:2015) is identical to its IEC counterpart with exception of references to the EN versions of the 61000-4-x series and the addition of an Essential Requirements annex.
Medical Design Standards-3 Edition Medtech Engine
Free Iec 60601 3rd Edition (PDF ePub Mobi)
10/07/2013 · MET will review information about the current status of medical product safety regulatory requirements. This is a complimentary broad overview that …
Introduction IEC 60601-1 (2 nd Edition) – published in 1988 is being replaced by: IEC 60601-1 (3 rd Edition) – published in 2005 Points of interest:
Download IEC 60601 2 57:2011 for FREE. All formats available for PC, Mac, eBook Readers and other mobile devices. Download IEC 60601 2 57:2011.pdf
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
60601- 1:2005, 3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of standalone and component power – supplies – Application of Annex B or Annex CAnnex C as applicable.
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
Page 2 iec 60601 medical design standards – 3rd edition www.cui.com standards are an integral part of product design and development, and are clearly important in…
3rd Edition or IEC 60601-1:2005 with Am. 1:2012, as applicable. 3.3 Additional considerations for certification of stand-alone and component power supplies – …
IEC 60601-1 Third edition 2005-12 This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French-language pages. Publication numbering As from 1 January 1997 all IEC publications are issued with a designation in the 60000 series. For example, IEC 34-1 is now referred to as IEC 60034-1
page 2 IEC 60601 Medical Design Standards – 3rd Edition www.cui.com Standards are an integral part of product design and development, and are clearly important in medical applications.
An expert discusses what medical device manufacturers need to keep in mind as the compliance date for the fourth edition of the IEC 60601-1-2 standard approaches.
•Major IEC 60601-1 3rd Ed. changes Transition to 60601-1, 3rd Edition (In the EU part 2 standards may complicate this, however) www.intertek.com Structure of IEC 60601 General standard (Part 1 standard) IEC 60601-1 Collateral standards IEC 60601-1-XX 60601-1-260601-1-3 60601-1-XX 80601-2-XX 60601-2-XX 60601-2-3 60601-2-2 60601-2-1 Particular standards (Part 2 standards) IEC 60601-2-XX
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
medicaL devices iec 60601 sgs.com
Be prepared for the 4th edition of the IEC 60601-1 medical
Features Software Verification and Validation The Role of
Public and private training to IEC 60601 2nd and 3rd editions, differences, ISO 14971, Amendment 1 and more Gap assessment to EN/IEC 60601 and ISO 14971 IEC 60601 Proactive Review – Early engagement for both 2nd and 3rd edition to support demanding project completion schedules.
CAN/CSA C22.2 NO. 60601-114 (R2018) techstreet.com
Risk Management in IEC 60601-1 3rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
Major IEC 60601-1 3rd Ed changes 9-14-10 PDF Free Download
Jeffrey A. Lenk President Professional Testing (EMI) Inc.
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
IEC 60601-1: Changes from 2nd to 3rd Edition – Intertek. The 3rd Edition of IEC 60601-1 represents a shift in philosophy from the 2nd acceptance of the 3rd Edition in the world’s largest markets for medical devices. gamma, neutron and other particle radiation; microwave radiation; visible.
Med-Info TÜV SÜD Global Website
What is the Scope of IEC 60601-12005 (3rd edition
Be prepared for the 4th edition of the IEC 60601-1 medical
TECHNICAL NOTE IEC 60601-1 3rd Edition + AM1 Guidelines for MOOP and MOPP Prepared by: Ross Sacolles 12/17/2013. TECNICAL NOTE I ls I Page 2 Class I: …
Features Software Verification and Validation The Role of
The TransiTion from The 2nd ediTion To The 3rd ediTion series At present choosing the edition that is the best fit for your device and markets of interest is a difficult decision.
Transition from 2nd to 3rd Edition of IEC/EN 60601-1
Features Software Verification and Validation The Role of
Major part of the content of the old IEC 60601-1-1 for IEC 60601-1, 2nd edition constitutes clause 16. RMF RMF Group of products, at least one of which is a MEE, functionally inter-connected or supplied from a Multiple Socket Outlet (MSO).
TECHNICAL NOTE 378m1o49xyfcl4may47q66uc
The numbering was revised to agree with IEC 60601-1:2005 (third edition). Beyond this, Beyond this, essential performance characteristics are defined in 201.4.3.101, guidance on maintenance is
Med-Info TÜV SÜD Global Website
Transition from 2nd to 3rd Edition of IEC/EN 60601-1
Iec 60601 1 3rd Edition.pdf Free Download – freebookee.com
An expert discusses what medical device manufacturers need to keep in mind as the compliance date for the fourth edition of the IEC 60601-1-2 standard approaches.
Major IEC 60601-1 3rd Ed changes 9-14-10 PDF Free Download
If your company needs help with IEC 60601-1 gap analysis, preparation of the risk management file for the third edition, or training on the Standard, please contact Leo Eisner at: Leo@EisnerSafety.com.
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
Iec 60601 1 First Edition.pdf Free Download
TECHNICAL NOTE 378m1o49xyfcl4may47q66uc
In 2005, the third edition of IEC 60601-1 was published. It was the result of a comprehensive review of the second edition (dating from 1988). Some key changes are: the outline and the numbering scheme of the clauses and subclauses were changed, risk management was made much more relevant and the concept of essential performance was added.
Free Iec 60601 3rd Edition (PDF ePub Mobi)
Review of IEC 60601-1-2 2014 (4th Edition) Interference
Medical Design Standards-3 Edition Medtech Engine
IeC 60601-1 evolutIon The IEC 60601 standard has a long history with a number of revisions. 2nd and 3rd edition standards must still coexist in equipment intended to ship internationally. creating an added level of complexity for device designers who must account for both standards. This is done because the product complexity generally yields a nearly uncountable number of potential test cases
EN 60601 Medical Electrical Equipment and Systems India
IEC 60601-1 Changes From 2nd To 3rd Edition.pdf Free
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment
TECHNICAL NOTE IEC 60601-1 3rd Edition + AM1 Guidelines for MOOP and MOPP Prepared by: Ross Sacolles 12/17/2013. TECNICAL NOTE I ls I Page 2 Class I: …
Overview of 60601 1 3rd Edition Webinar YouTube
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. Buying ready-qualified power supplies is one way to be prepared for the introduction of new standards.
Choices – IEC 60601-1 3rd Edition and Component Selection
IEC 60601 4th Edition Standards JADAK
IEC 60601-1 Ed. 3.1 en2012 Medical electrical equipment